The origin of cement
Cement is a very familiar building material in life. Modern city construction is inseparable from cement, but do you know the history of cement? It may be much longer than you think. As early as 5000 years ago, cement appeared. It is said that it was invented by the Mesopotamians. The main components are lime, sand and gravel. In the period of ancient Rome, the ancient Romans got inspiration from the natural formation of volcanic ash tuff, and roasted volcanic ash with lime and gypsum. This is the early cement. In 1824, the Englishman Joseph Aspuddin applied for a cement patent. Later, cement technology continued to develop, and in 1849 (also said to be 1850), the Frenchman Joseph Monier invented reinforced concrete. (Actually, he is a gardener, using wire in concrete to make a flower pot~)

Now, there are more than 100 varieties of cement in the world, which is one of the main building materials and plays an important role in social development and economic construction. However, during the sintering process of cement, a certain amount of fluorine-containing ore will be added. If the amount of calcium fluoride is too high, it will cause cement retardation, affecting strength and even gelation performance; if the content of sulfur trioxide exceeds the standard, it will cause cement The volume expands and destroys the cement structure, which affects the setting time and strength of the cement. In addition, the chlorate in the cement is brought into the concrete, which also causes corrosion of the steel bars.
Therefore, the detection of anions in Portland cement is of great significance. The pretreatment using ion chromatography conductivity method is simple, and the fluorine, chlorine and sulfate in the Portland cement can be tested in 10 minutes at a time.
1.Sample Preparation
Weigh about 1g of sample, put the sample in a 200mL beaker, add 50mL of water, stir until completely dispersed, add 1mL of nitric acid under stirring, heat to boiling, and boil for 5 minutes after cooling to room temperature, transfer the solution to centrifuge tube, centrifuge, transfer the supernatant to a 500mL volumetric flask, dilute to the mark with water, and mix. The above solution was passed through an IC-H column and a 0.22 μm needle filter, tested on the machine, and a blank experiment was conducted.
2.Instrument configuration and chromatographic conditions
This experiment uses two carbonate system columns SH-AP-2 and SH-AP-3 developed by SHINE in 2019, of which SH-AP-2 is a conventional carbonate system column, SH-AP-3 is a carbonate system fast column. The following is the instrument configuration and conditions of the two columns:
Instrument configuration:

Instrument: CIC-D150
Suppressor:SHY-A-6
Detector: conductivity detector
Chromatographic conditions

Column: SH-AP-2
Eluent: 8.0mmol/L Na2CO3 + 4.0mmol/L NaHCO3
Flow rate: 1.2mL/min
Detection method: suppressed conductivity method
Injection volume: 25uL
Column pressure: 9.8Mpa
Column temperature:35℃
Column: SH-AP-3
Eluent: 2.4mmol/L Na2CO3 + 3.6NaHCO3
Flow rate: 1.2mL/min
Detection method: suppressed conductivity method
Injection volume: 25uL
Column pressure: 9.8Mpa
Column temperature:35℃
Select the standard solution for the resolution test of the three ions. The test spectrum of the SH-AP-2 and SH-AP-3 standard products is as follows:
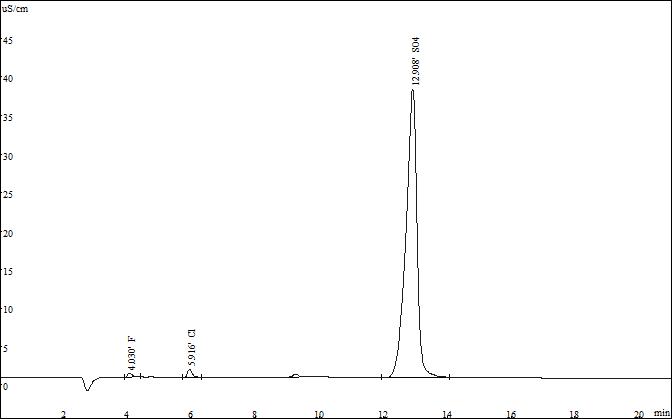
▲ Standard spectrum of SH-AP-2
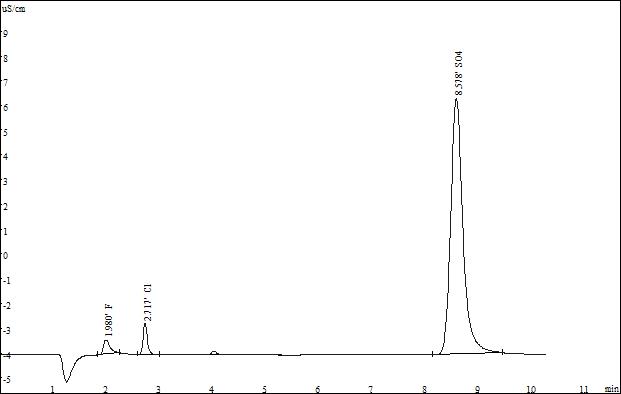
▲Standard spectrum of SH-AP-3
After testing, SH-AP-2 and SH-AP-3 columns can meet the test requirements, but the SH-AP-3 carbonate fast column can complete the three anion test within 10 minutes, so it is recommended to choose the preferred one.
3. Linearity and spectrum
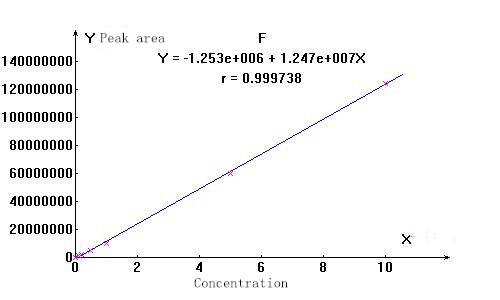
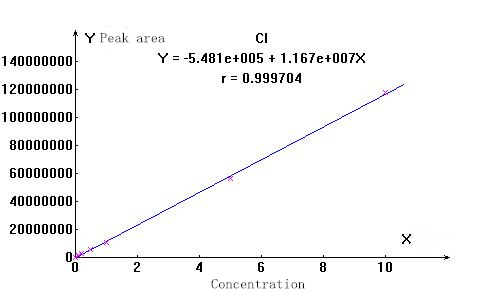
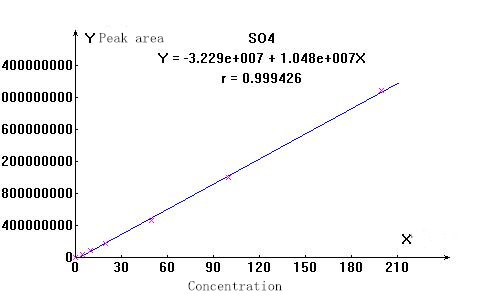
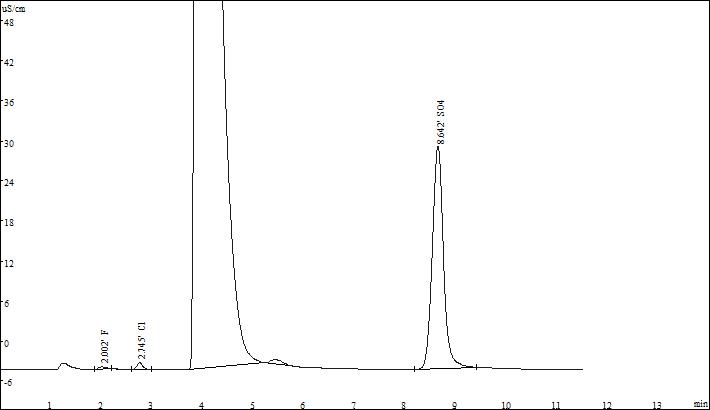
▲Actual sample spectrum
The stability of the ions to be measured is good, and the linear correlation coefficients are above 0.999. The separation effect of the actual sample from other impurity ions is good, which can meet the test requirements.