Tokyo Institute of Technology, in cooperation with SHINE, published a paper entitled Structures of Ions Accommodated in Salty Ice Ih Crystals in the Journal of Physical Chemistry Chemical Physics( PCCP).
Abstract
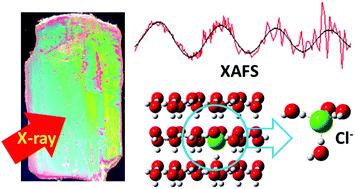
Frozen aqueous electrolytes are ubiquitous and involved in various phenomena occurring in the natural environment. Although salts are expelled from ice during freezing of aqueous solutions, minor amounts of the constituent ions are accommodated in the crystal lattice of ice. This phenomenon was associated with the generation of the Workman–Reynolds freezing potential. Molecular simulations also confirmed the ion incorporation in the crystal lattice of ice Ih upon freezing of aqueous electrolytes and identified possible local structures of the ions. However, no experimental information is available on the structure of ions accommodated in the crystal lattice of ice Ih. In this work, we use X-ray absorption fine structure (XAFS) to study the local structures of K+ and Cl− accommodated in ice Ih single crystals. Previous molecular simulations predicted that ions are trapped in the hexagonal cavities of the ice structure or replace two water molecules in the crystal lattice. Four possible configurations are considered and optimized by the calculations using ONIOM (QM/QM/QM). The results are evaluated in terms of the agreement between the experimental XAFS spectra and those simulated from the optimized structures. The spectra are most reasonably interpreted by assuming that K+ replaces one water molecule in the ice crystal lattice and is accommodated in a tetrahedral coordination cage. Similarly, Cl− probably adopts the same configuration, because it explains the coordination number better than other structures, such as that assuming the replacement of two water molecules belonging to the same hexagonal planes.
It used SHINE's CIC-D120 ion chromatograph, SH-CC-6 cation column and SH-AP-2 anion column.


For details, please click the link to view and download:https://pubs.rsc.org/en/content/articlelanding/2021/CP/D1CP01624E.

About PCCP

Physical Chemistry Chemical Physics (PCCP) is an international journal for the publication of cutting-edge original work in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and the Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
PCCP is proud to be a society journal and is co-owned by 19 national chemical societies. The journal is published by the Royal Society of Chemistry on a not-for-profit basis for the benefit of the whole scientific community.